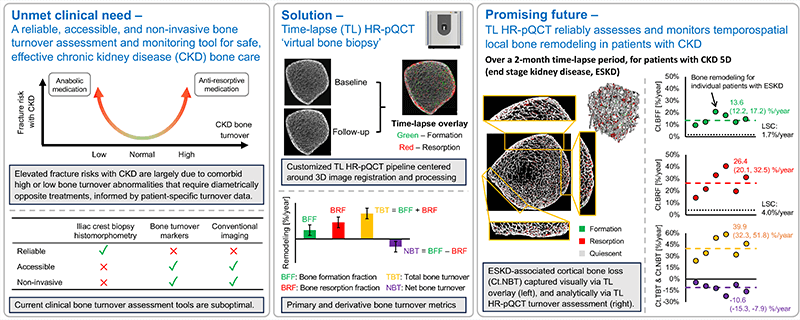
Patients with chronic kidney disease (CKD) have exceptionally high fracture risk. Medical management of fracture risk in CKD requires assessment of bone turnover. Bone turnover assessment is important because either high or low bone turnover can increase fracture risk, but treatment in these two bone turnover scenarios are diametrically opposite. There are now FDA approved medications that can either decrease (e.g. bisphosphonates) or increase (e.g. teriparatide) bone turnover, but these must be used carefully in CKD to avoid exacerbating fracture risk.
The gold standard measurement of bone turnover in CKD requires bone biopsy and histomorphometry, which is invasive, expensive, and not widely available. Thus, a particularly important clinical need is to identify novel methods to assess bone turnover rates non-invasively in CKD patients.
High resolution peripheral quantitative computed tomography (HR-pQCT) can image bone in fine detail with low radiation dose. We propose non-invasive ‘virtual bone biopsy’ for assessment of CKD turnover metrics through the application of time-lapse HR-pQCT. In time-lapse HR-pQCT, data obtained serially over time are processed to identify and quantify specific areas of new bone formation and resorption, enabling the direct measurement of bone formation rate, bone resorption rate, and total bone turnover.
We aim to compare time-lapse HR-pQCT to the gold-standard, tetracycline labeled histomorphometry by biopsy. We will also determine the sensitivity and specificity of time-lapse HR-pQCT for assessment of turnover status in a CKD population and determine whether time-lapse HR-pQCT can monitor changes in turnover in response to therapy. The data generated here will be used to design follow-on clinical trials testing whether treatment guided by time-lapse HR-pQCT can decrease fracture risk compared to usual care in CKD patients.
Co-PIs Joachim Ix, MD & Thomas Nickolas, MD
Funded by NIH/NIAMS R01 AR084815