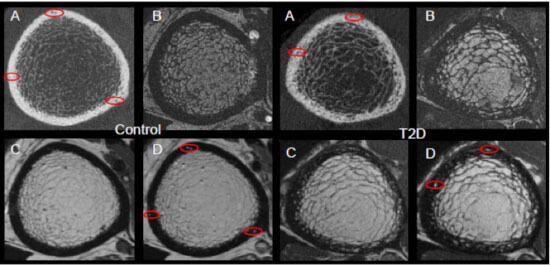
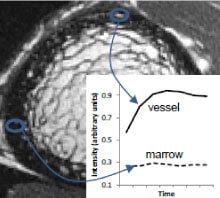
Patients with type 2 diabetes (T2D) have an increased risk for fragility fractures despite normal or even elevated bone mineral density (BMD) Recent findings suggest that diabetic bone exhibits abnormalities in bone quality, including elevated cortical porosity. Cortical porosity has deleterious effects on bone strength, and is critical in fracture initiation and propagation, but the origins and temporal evolution of pathological cortical porosity in T2D are unknown. To develop treatments specifically targeted to the prevention or reversal of pathological cortical porosity and associated bone fragility in T2D, we must understand the mechanisms driving development of these cortical pores. The goal of this study is to investigate the underlying biological processes that drive increased cortical porosity in the setting of T2D and to understand the longitudinal evolution of human diabetic bone disease with a special focus on cortical porosity. We are currently conducting a longitudinal study of pore progression in T2D patients, using a novel combined high-resolution peripheral quantitative computed tomography (HR-pQCT) and dynamic contrast enhanced magnetic resonance imaging (DCE MRI) approach. We are developing novel image analysis approaches to characterize pore content and spatial distribution of porosity within the cortex, and are using micro finite element (μFE) analysis to quantify the biomechanical impact of porosity.
Funded by NIH/NIAMS R01 AR069670