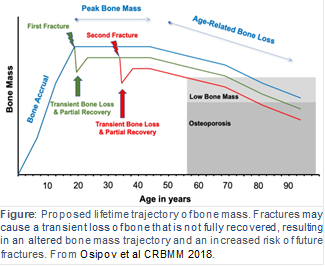
Osteoporosis and low bone mass affect over 50 million Americans, and osteoporosis-related fractures will account for over three million broken bones and $25 billion in related costs each year by 2025. Identifying the underlying causes of bone loss are therefore of utmost importance for improving the skeletal health of a rapidly aging population. Epidemiologically, the most reliable predictor of fracture risk is a history of fracture at any skeletal site. The etiology of this relationship is not fully known and depends on many factors (e.g., low bone mass, poor bone quality, risk of falls), but one underappreciated mechanism is that fracture initiates systemic bone loss throughout the skeleton, which increases future fracture risk at all skeletal sites. Our lab has generated multiple preclinical studies characterizing this post-traumatic systemic bone loss response following fracture in mice. However, post-traumatic bone loss following fracture has never been specifically investigated in human subjects. Therefore, the long-term goal of this research is to address this knowledge gap by determining the time course and magnitude of post-fracture bone loss and recovery in human patients and to determine mechanisms driving this response (e.g., systemic inflammation, decreased activity level, mineral utilization), and eventually to evaluate treatments aimed at preserving skeletal health following fracture in older patients.
In the current project, we use standard clinical and cutting-edge high-resolution imaging to characterize the time course and magnitude of systemic bone loss and recovery following humerus fracture in older patients. Based on currently available clinical data, we hypothesizethat post-fracture systemic bone loss: 1) will persist for 6 months or more post-fracture, 2) will have a greater effect on trabecular bone than cortical bone, and 3) will be greater in patients that exhibit increased post-fracture systemic inflammation.
Co-PI Blaine Christiansen, PhD
Funded by NIH/NIA R01 AG078347