Recent FDA approvals (e.g. Lutathera, Pluvicto) and a swell of promising experimental agents in clinic trials underlie a surging enthusiasm for radioligand therapy (RLT) as a treatment modality in many cancers. However, clinical experience also shows that tumor responses to RLTs are often transient and/or variable among patients. Thus, there is a pressing need for improved understanding and new strategies that maximize the therapeutic benefit of RLT for cancer patients.
For the past decade, the nuclear medicine field has prioritized developing low molecular weight small molecule, peptide, or biologic RLTs that rapidly exit the bloodstream, with the objective of minimizing host toxicity. Despite rapid clearance, small RLTs can effectively treat tumors if they potently bind a highly overexpressed tumor protein (e.g. PSMA, SSTR2, FAP, GRPR). However, RLTs targeting endogenous ligand binding pockets on cell surface receptors co-opt a signaling mechanism that is evolutionarily designed to be transient, and thus ligand dissociation and/or receptor degradation limits the lifetime of ligand–receptor interactions. Thus, the current state of the art likely does not fully maximize the antitumor effects of isotopes like Lu-177 and Ac-225, which have half-lives that span many days to even weeks. On this basis, developing new chemical strategies to deliver and retain radioisotopes in tumors is a meritorious goal.
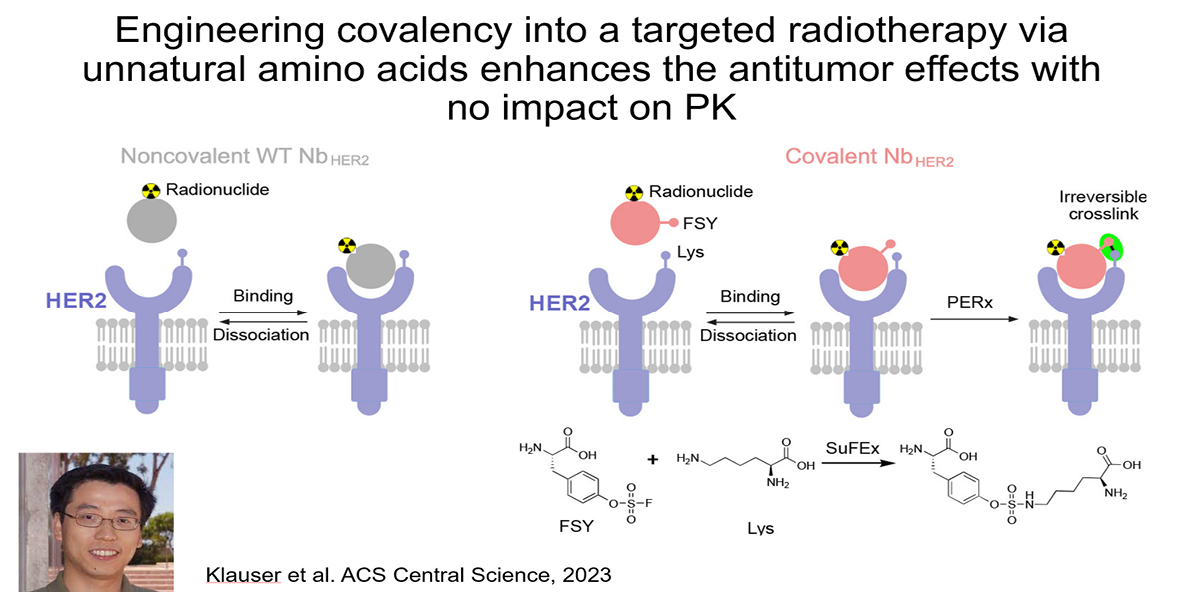
Addressing this challenge is a major emphasis of the lab. Recently, we showed that installing covalency into an RLT can increase tumoral absorbed dose without impacting the overall pharmacokinetic profile of the therapy. Using an anti-HER2 nanobody, we collaborated with Lei Wang’s lab at UCSF to show that installing a lysine reactive unnatural amino acid triggered covalent crosslinking with cell surface HER2 on human xenografts in mice. Crosslinking increased tumoral AUC compared to the noncovalent nanobody by eliminating ligand/receptor dissociation (i.e. koff) thereby extending the retention time of the isotopic payload in tumors. The 225Ac-labeled covalent nanobody more potently suppressed xenograft growth compared to the wild type, non-covalent antibody, as expected. Notably, the PK profile of the mutant nanobody was not significantly impacted by the presence of a mildly reactive electrophile. This is one of several strategies we are pursuing in collaboration with others like Drs. Adam Renslo, Kevan Shokat, Charly Craik, and Jim Wells.

Relevant publications:
- Proteins as Targeted Radionuclide Therapies Enhance Antitumor Effects. ACS Cent Sci. 2023 Jun 28; 9(6):1241-1251.Klauser PC, Chopra S, Cao L, Bobba KN, Yu B, Seo Y, Chan E, Flavell RR, Evans MJ, Wang L. PMID: 37396859; PMCID: PMC10311652.