The recent success of inhibitors against immune checkpoint proteins (e.g. CTLA-4, PD-L1), which are thought in part to stimulate T cell responses against tumors, and chimeric antigen receptor (CAR) T cells has revolutionized cancer therapy. Yet only ~20-30% of patients achieve deep responses, and discerning responders from non-responders is challenging with conventional imaging. Thus, there is an urgent unmet need to develop new imaging biomarkers to distinguish responsive and resistant patients, as well as to detect undesired immune related adverse events.
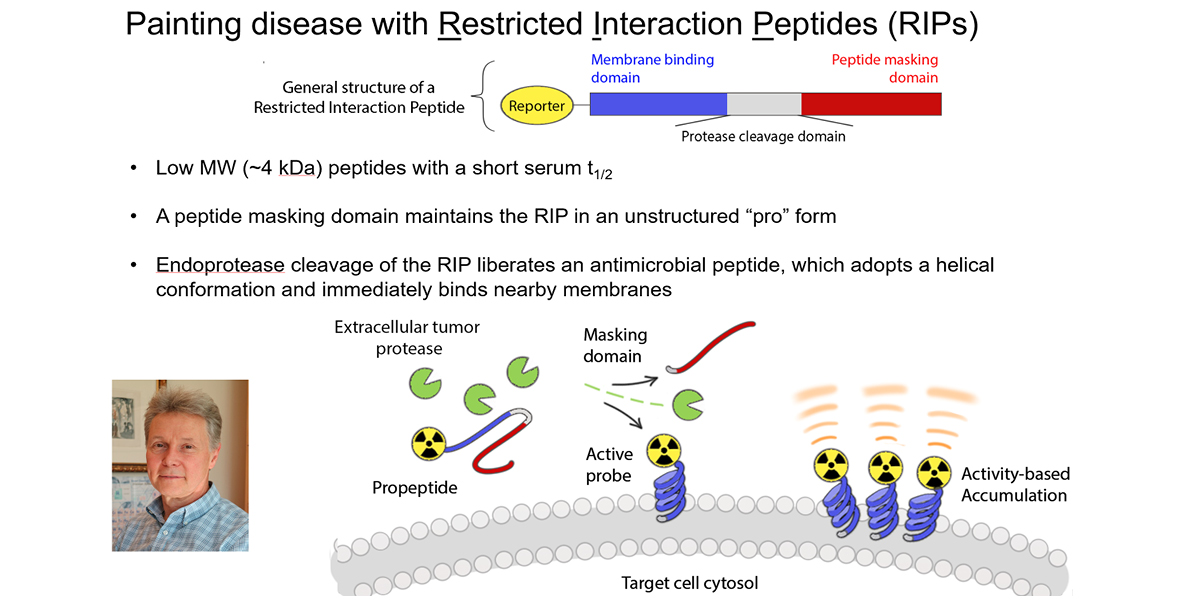
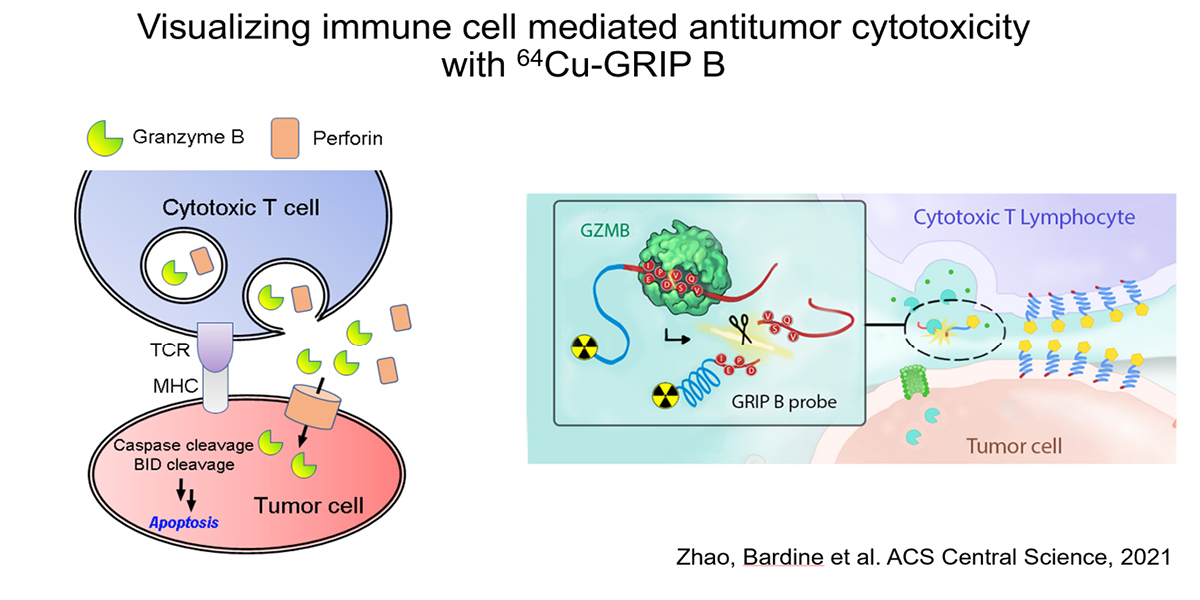
We hypothesized that an imaging technology capable of selectively measuring the biology utilized by T cells to impart cytotoxicity could predict early tumor responses. Since T cell cytotoxicity against tumor cells is primarily conferred by the pro-apoptotic serine protease granzyme B (GZMB), we have developed a novel probe termed GRIP B that measures the biochemistry of GZMB as it traverses the pericellular space at the immunological synapse. GRIP B is modeled after a “restricted interaction peptide”, a technology that we recently pioneered to enable the first spatiotemporal measurements of endoprotease biochemistry in vivo with PET. Mechanistically, an inactive “pro-form” of radiolabeled GRIP B is administered systemically, whereupon cleavage by GZMB releases a radiolabeled (non-toxic) antimicrobial peptide that spontaneously adopts a helical conformation to immediately associate with nearby phospholipid membranes. Thus, sequestration of the radiotracer by the endoprotease provides a readout of the relative units of enzyme activity within a region of interest. The specificity of GRIP B is driven by the biochemistry of GZMB, which is the only known extracellular protease in humans that can cleave a peptide with an aspartic acid at the P1 site.
Our preclinical data in mouse cancer models show that 64Cu-labeled GRIP B detects granzyme B from T cells activated with anti-PD-1 and anti-CTLA4 therapies. Provocatively, the radiotracer detects proteolytic activity within tumors, but also among normal tissues (e.g. the spleen) where immune cells would be exposed to systemic immune checkpoint inhibition, which are known to induce immune-mediated toxicities. We confirmed the specificity of 64Cu-GRIP B for GZMB in vivo by conducting imaging studies in tumor bearing mice with germline GZMB knockout, and by applying an uncleavable, D amino acid adduct of 64Cu-GRIP B. Lastly, we have shown that post treatment tumoral uptake of 64Cu-GRIP B is inversely correlated with volumetric tumor responses to checkpoint inhibitors in the wild type, but not GZMB-/- mouse background. We recently opened a clinical trial at UCSF to test 64Cu-GRIP B as a biomarker of response to immunotherapies (NCT05888532).
Relevant publications:
- In Vivo Measurement of Granzyme Proteolysis from Activated Immune Cells with PET. ACS Cent Sci. 2021 Oct 27; 7(10):1638-1649.Zhao N, Bardine C, Lourenço AL, Wang YH, Huang Y, Cleary SJ, Wilson DM, Oh DY, Fong L, Looney MR, Evans MJ, Craik CS. PMID: 34729407; PMCID: PMC8554823.